Published on August 25, 2020
Decoration of γ-Graphyne on TiO2 Nanotube Arrays: Improved Photoelectrochemical and Photoelectrocatalytic Properties
Applied Catalysis B: Environmental
Keywords: graphyne , photocatalysis , photoeletrochemistry , TiO2 nanotube arrays
Abstract
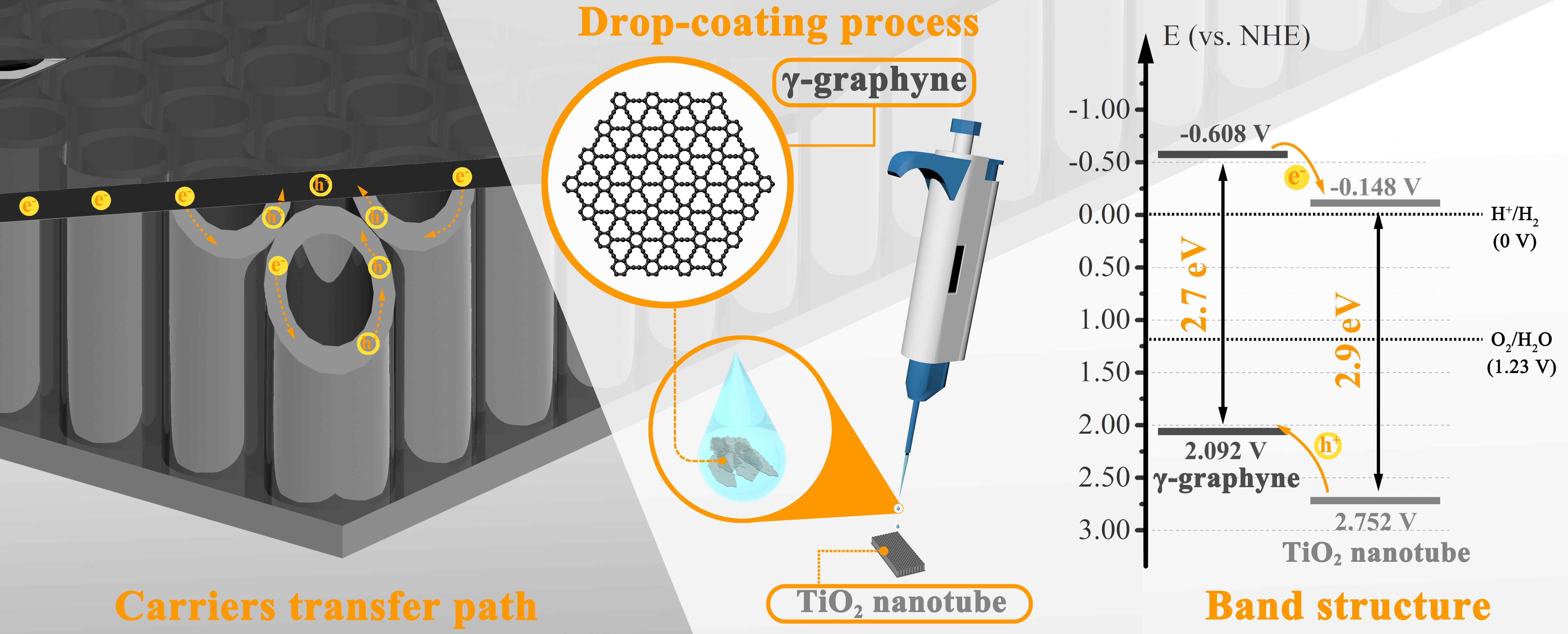
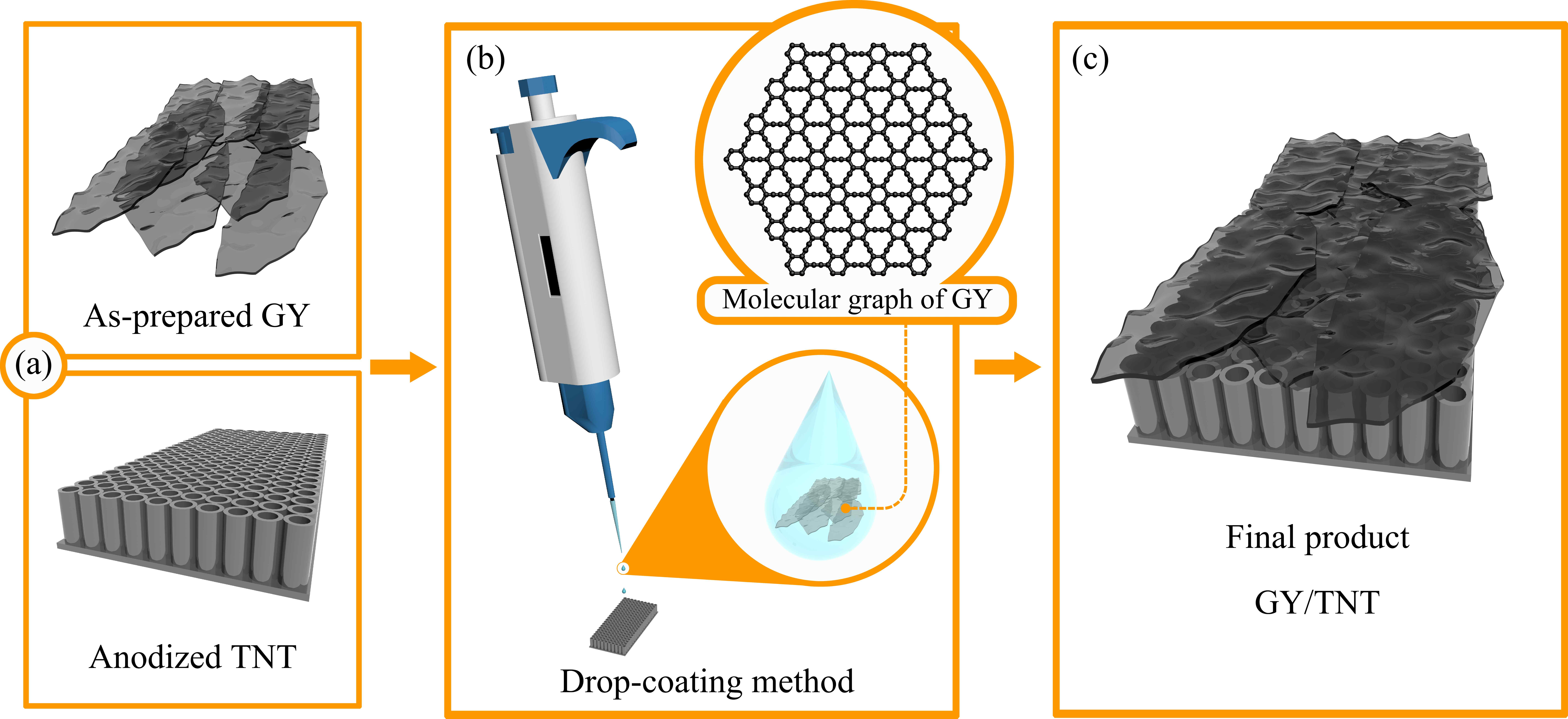
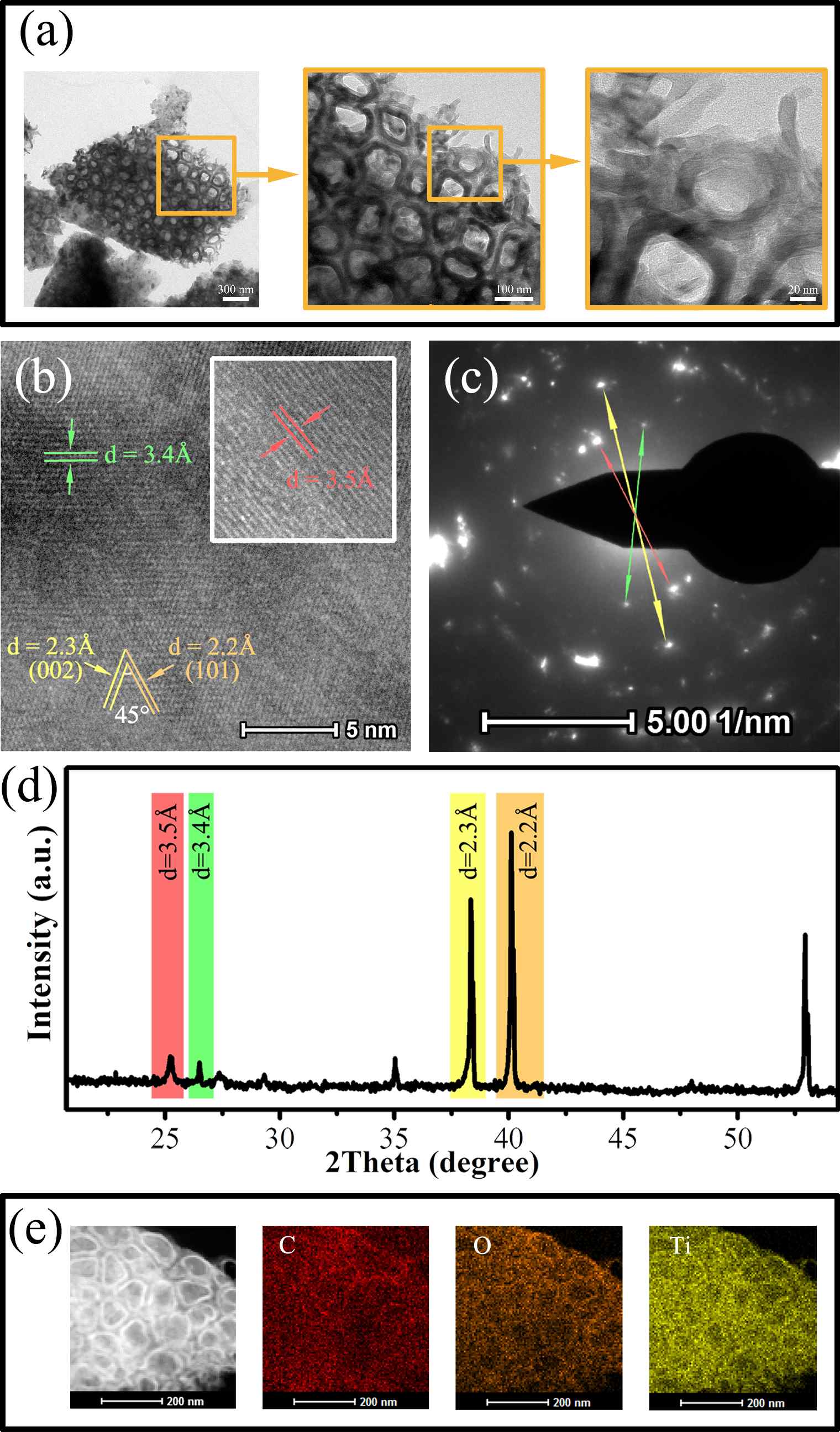
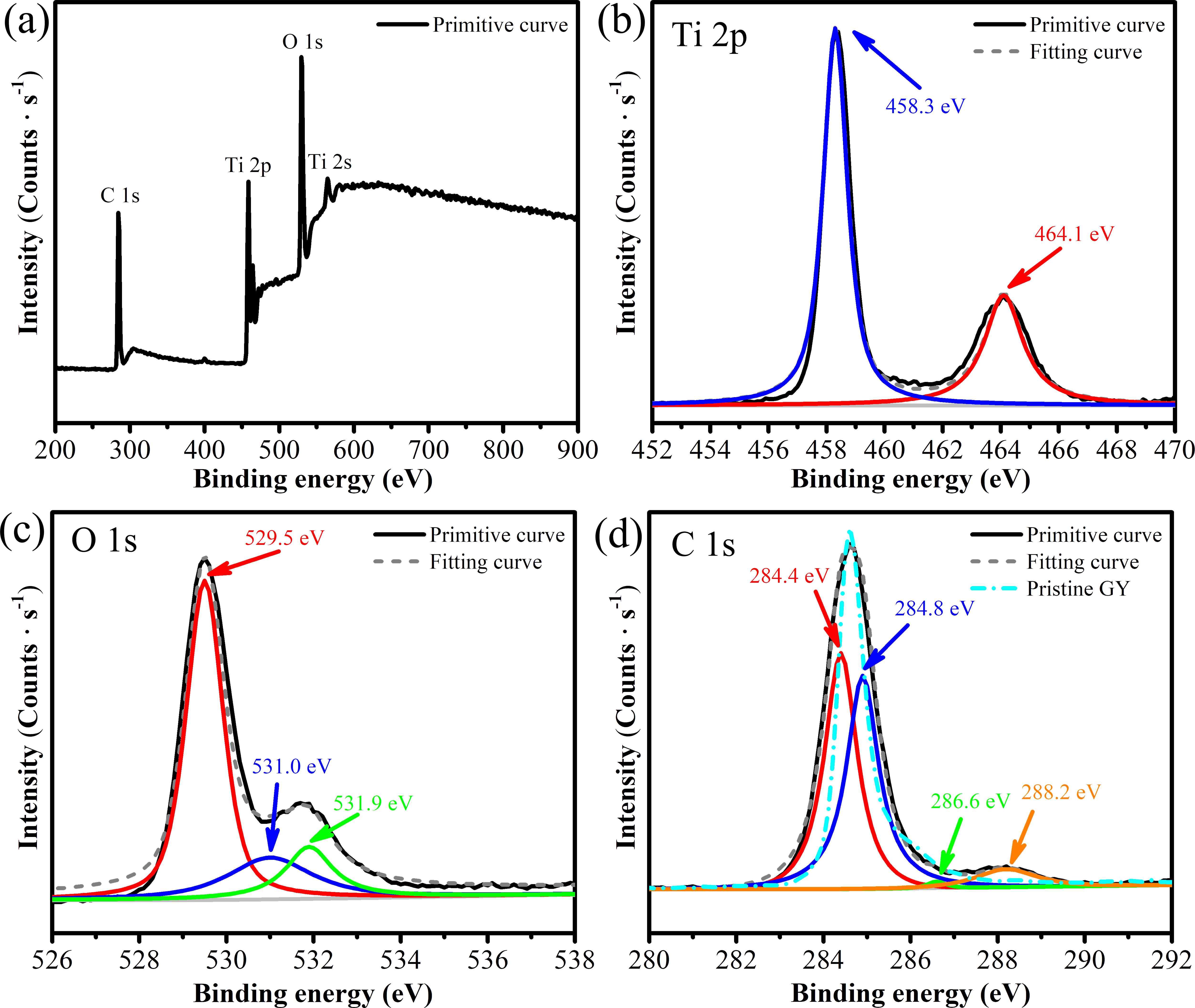
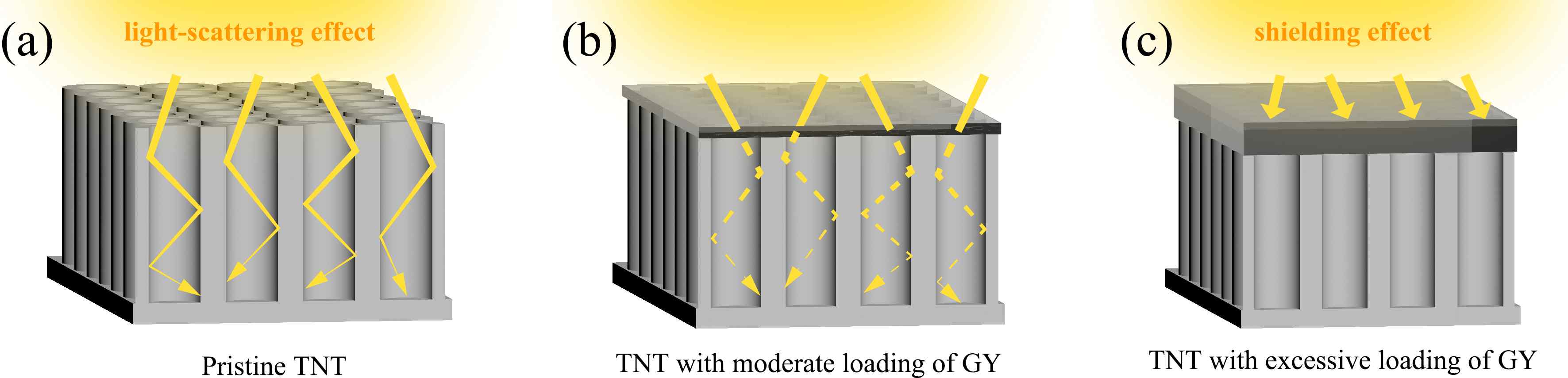
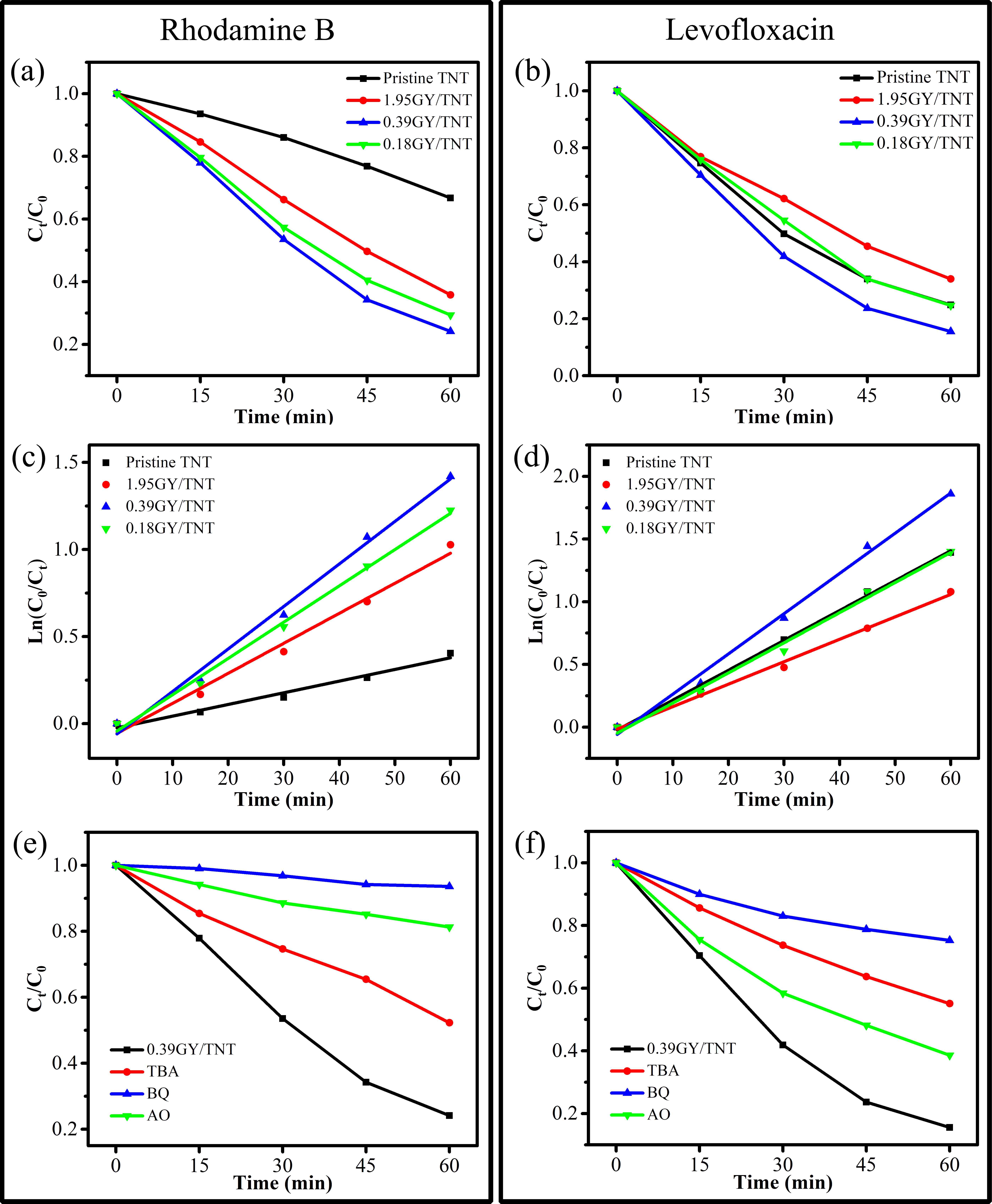
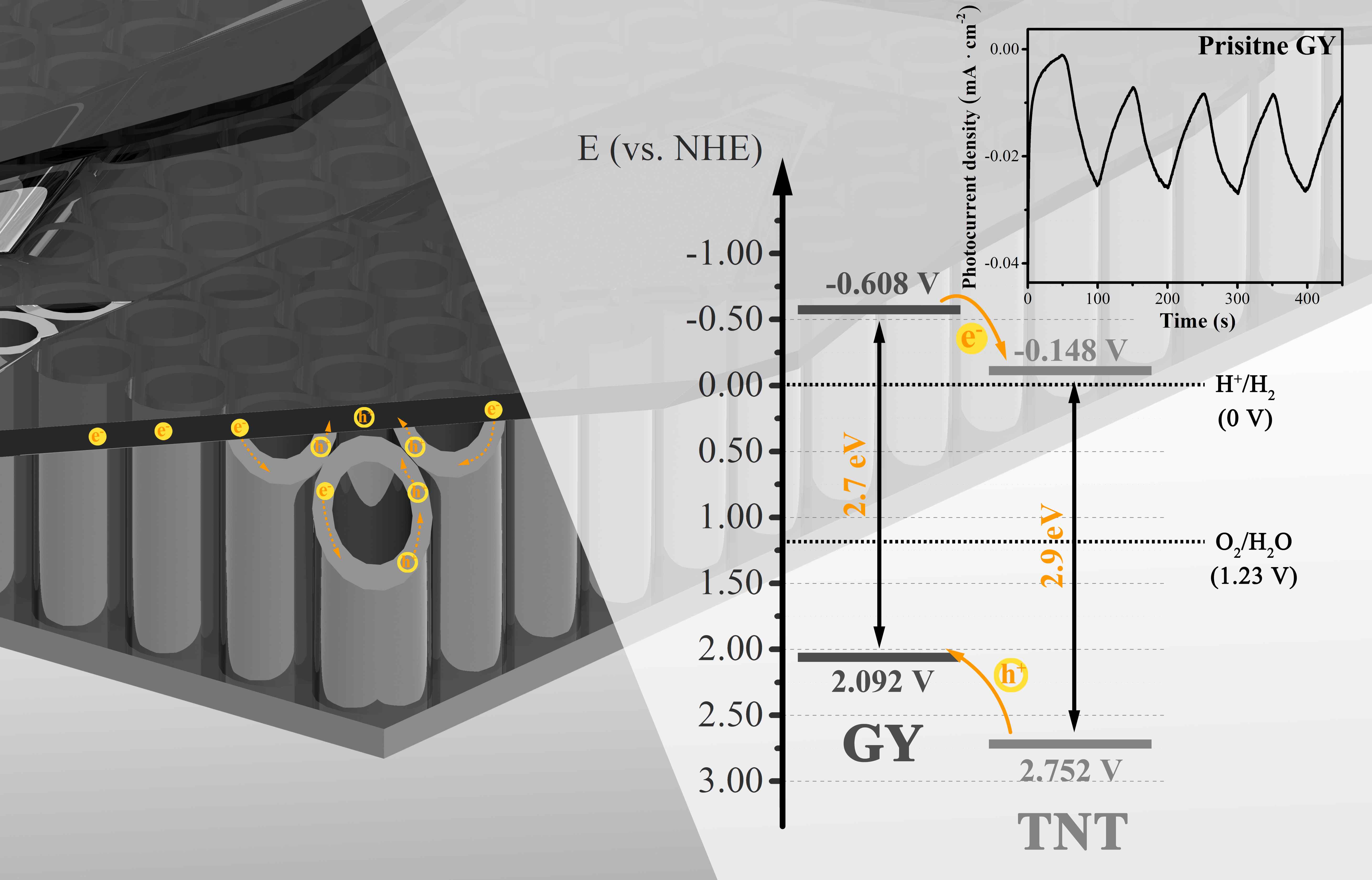
A series of γ-graphyne/TiO2 nanotube array heterostructures are first synthesized by a facile and environmentally friendly drop-coating method. The prepared heterostructures are completely investigated by a collection of characterizations. Interestingly, the unique C-O-Ti linkage between γ-graphyne and TiO2 nanotube arrays is verified by both X-ray photoelectron spectroscopy and Fourier transform infrared analysis. After modification of γ-graphyne, the maximum transient photo-current and photo-potential of TiO2 nanotube arrays are improved by 2.2 and 1.3 folds, respectively. Moreover, a maximum of 3.64 and 1.35 folds enhancements are severally verified for the photoelectrocatalytic degradation of levofloxacin and rhodamine B over the heterostructures compared to that of TiO2 nanotube arrays. Furtherly, the heterostructures also show superior photoelectrocatalytic performance on nitrogen fixation and oxygen evolution than TiO2 nanotube arrays. The crystal, morphological, photoelectrochemical, and photoelectrocatalytic stability of the heterostructures are confirmed. This work sheds light on designing γ-graphyne modified composites for photoelectrochemical and photoelectrocatalytic application.